
Specifications
| Product name | Electrical Stimulator WILMO |
|---|---|
| Regulations | PMDA (Japan) and TFDA(Taiwan) |
| Certification number (JAPAN) | 230AFBZX00063000 |
| Class category | Class IIa |
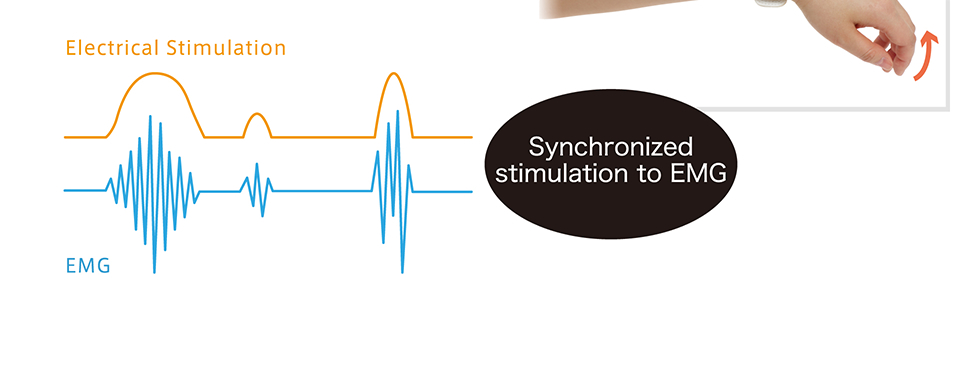
| Purpose of use | This product is used to stimulate nerves and muscles to provide transcutaneous pain relief and improve muscle atrophy. |
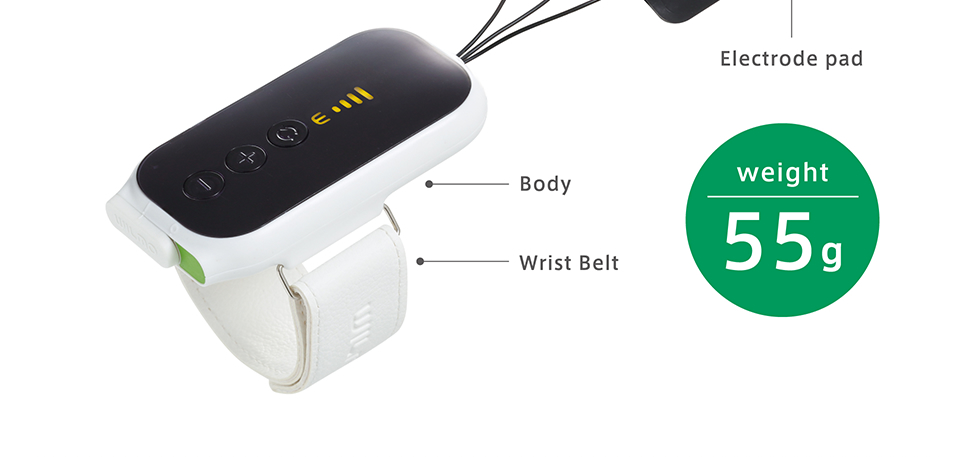
| Size | 49 x 98 x 14.5mm (except the wristband) |
| Weight | 55g |
| Frequency | 20Hz±10% |
| Treatment time | Max 8hours |
| Compatible standards |
IEC 60601-1: 2005+AMD1: 2012 IEC 60601-1-2: 2014 IEC 60601-2-10: 2012+AMD1: 2016 |
| Driving time | Max 8hours |
| Charging time | Max 3hours |
* Specifications subject to change.
WILMO is a registered trademark of SK-Electronics CO., LTD., Kyoto Japan.
WILMO was developed under the instruction of Dr. Yoshihiro Muraoka, former Lab Direction at the National Hospital Organization Murayama Medical Center and professer in the Department of Health Sciences and Social Welfare at the School of Human Sciences, Waseda University Pat. No. 5725562: Electrostimulation device (Compact electrostimulation circuit that can detect EMG signals)
Technology transferred from Human Science Technology Transfer Center, a TLO certified by the Minister of Health, Labour and Wealfare.










